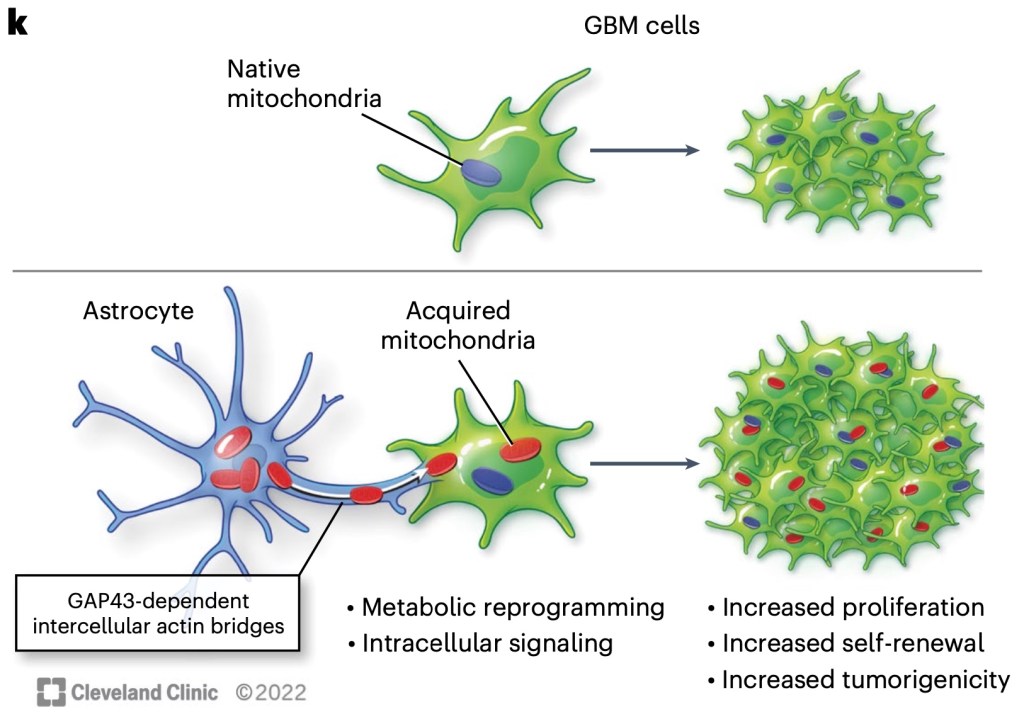
To proliferate and self-renew, cells of glioblastoma (GBM) need significantly faster metabolism than their ancestral neuronal cells, and for that, they take mitochondria from glial cells, mostly from astrocytes. This is achieved by “hijacking” the healthy mechanism of astrocyte reactivity: growth factor GAP43 produced by GBM cells stimulates astrocytes to produce microtubes that connect their cytoplasm and cytoskeleton with GBM cells. In turn, mitochondria move from astrocytes to GBM cells, and additional microtubes spread these mitochondria to GBM cells with no direct contact with astrocytes.
Recent paper of Watson et al. from Cleveland OH, Bergen (Norway) etc. shows new details of this process, in particular the role of GAP43. The migration of mitochondria was traced by xenografting human glioblastoma cells to brains of mice in which mitochondria expressed a red fluorescent protein, and also with in vitro models. This is a striking example how much tumor metabolism can be altered by its micro-environment, and of cooperative behavior of the tumor cells. The necessary role of GAP43 expression was proven with GAP43 knockdown in GBM prior to the construction of a model.
Watson, D.C., Bayik, D., Storevik, S. et al. GAP43-dependent mitochondria transfer from astrocytes enhances glioblastoma tumorigenicity. Nat Cancer 4, 648–664 (2023).