Fukaya et al (Cell Reports 4/25/2023) investigate signaling between leukocytes in the gut with healthy biome, and with dysbiosis, and a mechanism that perpetuates intolerance of beneficial microbes and food allergies. To a non-specialist, this is a fascinating window to immune system, multiple cell types and the intricate system that results in decisions: friend or foe? Correctly identified foes are pathogenic infections, tumor cells etc. that should be eliminated, and are eliminated most of the time, but wrongly identified foes lead to inflammatory diseases, reduced absorption of nutrients and to allergies, including asthma. Identification of cell types and signaling molecules that are involved may lead to more effective therapies for widespread diseases, and improve therapies that induce dysbiosis: like antibiotics and immunotherapy of cancers.
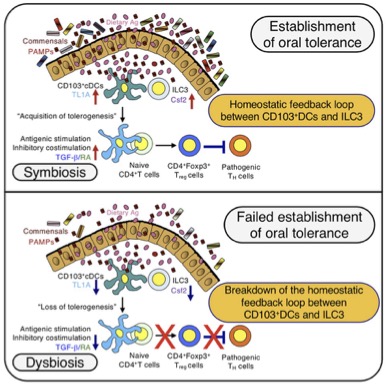
The title of this paper is “Gut dysbiosis promotes the breakdown of oral tolerance mediated through dysfunction of mucosal dendritic cells”, and “as everybody knows” (but I have learned after reading), oral tolerance is the desirable tolerance of foods and bacteria in the gut (not in the mouth). The cells that are advocates of suspect antigens (signaling that they should be tolerated) are a subset of conventional dendritic cells with CD11c and CD103 markers. In the aftermath of dysbiosis induced by antibiotics (and perhaps by wrong nutrition etc.), the proportion of these cells decreases, and so does the tolerance, and rejection of beneficial bacteria may perpetuate this unhealthy situation.
The discoveries reported in this paper include more refined subtyping of the cells involved in this process and many signaling interactions and feedbacks. I learned that the dendritic cells display antigen and instruct CD4 T-cells to become Tregs, T-cells that stop inflammatory reactions to the “approved” antigens. Recovery from dysbiosis and prevention may rely on common sense approaches like diet change and probiotics, but because of the feedback loop described in the paper, we may need to pharmacologically inhibit some signaling molecules while minimizing negative effects. And the more we know about this signaling, the better choices we can make.